Advertisement
Non-invasive blood glucose monitoring by NIR spectroscopy has evolved over decades as a promising alternative to finger-prick methods. However, despite significant research, regulatory approval remains elusive. Recent clinical studies, such as MDPI’s evaluation of the Glucube® NIR device involving 60 adult participants and 1,500 paired measurements, demonstrated a total MARD (Mean Absolute Relative Difference) within zones A and B at ~99.26%, suggesting clinical relevance—but regulatory standards explicitly exclude non-invasive formats.
Major consumer electronics companies (e.g., Samsung, Apple, Rockley Photonics) are actively developing Raman and NIR-based wearables. While the FDA warns against premature claims, these efforts reflect rapid progress even amid FDA’s caution.
Also Read: Continuous Non-Invasive Blood Glucose Measurements: Present Situation (May 2025)
Principles of NIR Measurement
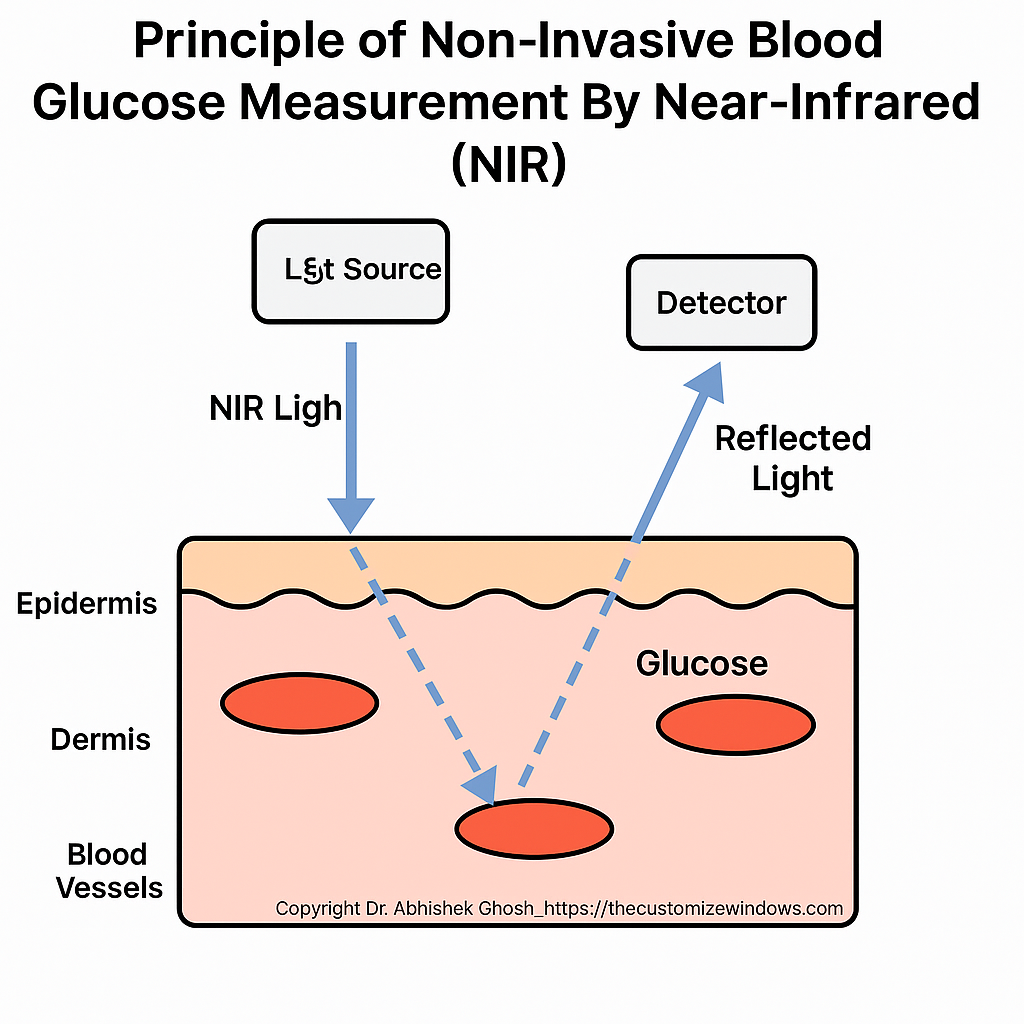
Spectroscopy Fundamentals
NIR relies on overtone and combination vibrational bands of glucose’s C–H, O–H, and C–O bonds within the 700–2500 nm range. Instruments use pulsed or continuous NIR light sources (LEDs or narrowband lasers) and sensitive thermal or photodiode detectors to capture light after tissue interaction.
Light–Tissue Interactions
NIR light undergoes absorption by water, glucose, lipids, and proteins, and scattering due to tissue microstructures. Variations in glucose concentration subtly alter the diffuse scattering coefficient, affecting both the intensity and path length of reflected or transmitted light.
Patent Landscape: Instrumentation & Measurement Strategies
Key Patents
- US 5086229A (1992, Rosenthal et al.): Introduced a handheld NIR unit (600–1100 nm) with source filter, detector, and processing electronics to quantify glucose through fingers—setting early foundations.
- US 5823966A (1998, Buchert): Advanced continuous NIR monitoring using spectrally selective emission and detection.
- US 9885698B2 (2018): Emphasized differential reflectance using dual probes to isolate vein from non-vein signals, mitigating skin variability.
- US 6097975A (2000, BioSensor): Applied narrowband light pulses and comparative filtering to enhance glucose sensitivity via reflection modes.
- EP 3747363A1: Described multi-wavelength NIR imaging using a finger-cradle and camera-based device for snapshot spectrometry.
Patent Insights
These patents underscore persistent themes: optimization of source wavelengths, differential measurement to reduce tissue interferences, and mechanical stabilization to ensure repeatable readings—collectively tackling core signal challenge issues.
Clinical Data & Instrument Performance
Glucube® MDPI Study
A June 2024 MDPI study deployed the Glucube® portable NIR device on 60 participants, capturing 1,500 measurement pairs across fasting, pre-/post-prandial, and nocturnal states.
Key performance metrics:
- Total MARD: ~99.26% readings in Clarke Error Grid Zones A+B.
- ISO15197:2015 compliance: Achieved across various glucose states.
- Algorithm stabilization: Performance improved after a week of adaptation.
iGLU Devices
Research from ArXiv introduced dual-wavelength NIR devices:
- iGLU 1.0: MARD ≈ 4.66%, AvgE ≈ 4.61%, R² ≈ 0.81
- iGLU 2.0: MARD ≈ 4.86% for serum glucose, demonstrating better precision than capillary measurement
Raman & MIR Hybrid Devices
Devices using mid-infrared quantum cascade lasers (e.g., DiaMonTech) reported:
- Mean percent difference: ~12.1%, median ~6.5%
- Zones A+B inclusion: ~99% of readings within clinical accuracy limits
Large-Scale Raman Trial
RSP Systems conducted a study with 160 diabetic patients and over 12,000 measurements:
- MARD: 14.3%, RMSE 1.9 mmol/L
- Clarke Error Grid: 96.5% in Zones A+B (Type 1 diabetics)
Technical & Clinical Challenges
Weak Signal Intensity: Glucose absorption is faint and overwhelmed by dominant absorbers like water and proteins.
Spectral Overlap: Requires multivariate statistical methods (PLS, ANN) to extract glucose signal from noise.
Physiological Variability: Factors like skin thickness, temperature, and hydration greatly influence readings.
Calibration Drift: Models degrade over time; adaptive calibration is essential.
Clinical Rigor: Current non-invasive devices still trail behind FDA-approved CGMs in reliability and robustness.
Future Directions: Emerging Technologies & Regulatory Outlook
Sensor Fusion & Machine Learning
Multi-sensor platforms combining NIR, MIR, Raman, and RF data with AI models show potential to overcome user-specific variability. Real-time drift detection and calibration adaptation using deep neural networks are emerging solutions.
Wearable OEM Interest
Companies like Apple, Samsung, and Rockley Photonics are filing patents and testing prototypes for smartwatches and rings with NIR/Raman-based glucose estimation features.
Mid-IR, Raman, and SPR
Techniques like photothermal MIR (DiaMonTech) and SPR-based nanophotonics (e.g., sweat-sensing) have demonstrated sub-3 mg/dL glucose sensitivity under lab conditions. Clinical translation remains in early stages.
Regulatory Standards
Non-invasive devices must meet ISO 15197 or FDA 510(k) standards for approval, which require sustained performance over time and error tolerances within ±15 mg/dL or 15% (depending on glucose range).
Conclusion
Near-infrared spectroscopy for non-invasive glucose monitoring has moved from theoretical groundwork to real-world feasibility. Although not yet commercially dominant, strong advances in dual- and multi-wavelength systems, wearable optics, and calibration methods are making rapid headway.
With continued clinical trials and AI-driven compensation for user-specific variability, NIR has a clear pathway toward reliable, pain-free glucose monitoring for millions of diabetics. Success, however, will hinge on meeting stringent regulatory standards and sustaining accuracy under real-world, longitudinal conditions.
Also Read: Best Large Language Models in Disease Diagnosis (2025): A Comprehensive Review